Exploring the World of Alloy Materials
You’ve probably heard of alloys before. But how much do you know about what makes them unique?
Pure metals are rarely strong enough for most practical applications. That’s where alloys come in.
Alloys are blends of metals, often mixed with non-metals. They’re designed to improve the properties of pure metals. Adding other elements creates characteristics the metal couldn’t achieve alone. These enhancements make alloys stronger, harder, and more durable.
At ProleanTech, we specialize in CNC machining services that maximize the potential of alloy materials. Our precision manufacturing ensures you benefit from these remarkable metal combinations.
Alloys are essential across industries. From aerospace to automotive, medical to construction – they’re everywhere in our daily lives.
This guide provides detailed information about alloy composition, properties, and usage. We’ll help you understand why alloys matter and how ProleanTech can help you leverage their unique benefits for your projects.
What is an Alloy Material?
Alloy has a metallic base, the major constituent, and some metal/non-metal components to modify the properties. Alloys meaning refers to materials that combine multiple metallic elements to create substances with enhanced properties compared to pure metals. Alloy metals are carefully designed to fulfil the purpose they are made for.
Here is a brief overview of alloy meaning, including composition, properties, and usage.
| Alloy Name | Composition | Properties | Uses |
| Steel | Iron (Fe) with 0.02-2% Carbon (C) and other elements like Mn, Si, Cr, and Ni. | High strength, hardness, moderate corrosion resistance, and good machinability. | Construction, automotive parts, tools, bridges, pipelines. |
| Aluminum Alloys | Aluminum (Al) with Cu, Mg, Mn, Si, Zn. | Lightweight, high ductility, corrosion-resistant, good thermal and electrical conductivity. | Aerospace components, automotive parts, packaging, and electrical conductors. |
| Brass | Copper (Cu) 60-70%, Zinc (Zn) 30-40%, sometimes with Pb or Sn. | Good corrosion resistance, excellent machinability, moderate strength, and attractive finish. | Musical instruments, plumbing fixtures, decorative items, electrical connectors. |
| Bronze | Copper (Cu) 85-95%, Tin (Sn) 5-15%, sometimes with Zn, Al, or Pb. | High wear and corrosion resistance, good thermal and electrical conductivity. | Bearings, bushings, ship parts, sculptures, electrical contacts. |
| Titanium Alloys | Titanium (Ti) with Al, V, or other elements. | High strength-to-weight ratio, excellent corrosion resistance, biocompatible, non-magnetic. | Aerospace, medical implants, chemical processing, marine applications. |
| Nickel Alloys | Nickel (Ni) 50-80%, with Cr, Fe, Mo, Cu, or Co. | Excellent corrosion and oxidation resistance, high strength, good creep resistance. | Turbines, jet engines, chemical processing equipment, high-temperature applications. |
| Inconel | Nickel (Ni) > 50%, with Cr, Fe, Mo, Nb. | Exceptional strength at high temperatures, excellent oxidation and corrosion resistance. | Jet engines, gas turbines, chemical processing, marine environments. |
| Pewter | Tin (Sn) 85-99%, with Cu, Sb, or Pb. | Soft, easily moldable, good corrosion resistance, low melting point. | Decorative items, jewelry, tableware, small sculptures. |
| Cast Iron | Iron (Fe) with 2-4% Carbon (C), Si, and Mn. | High compressive strength, good wear resistance, brittle. | Pipes, machine bases, cookware, automotive brake discs. |
| Lead Alloys | Lead (Pb) with Sb, Sn, or Cu. | High density, soft, excellent corrosion resistance (against certain chemicals). | Radiation shielding, batteries, roofing, corrosion-resistant linings. |
How are Alloys Made?
Formation of an Alloy: Step-by-Step Process
Alloys are made of mixing elements in a particular proportion to create a specific mixture. They are made by melting the base metal and alloying elements, mixing them, and then allowing the mixture to solidify once it cools down.
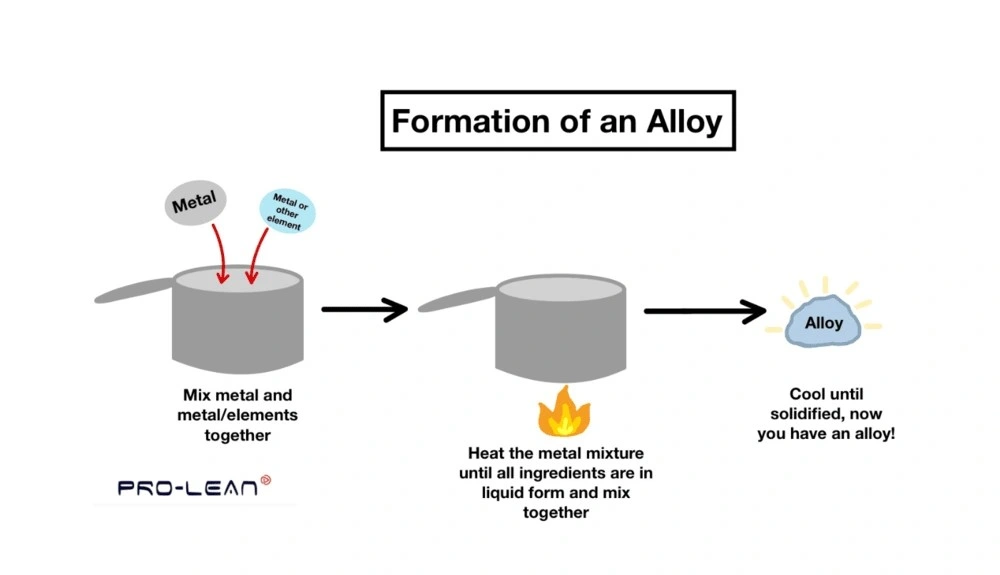
Are alloys mixtures or compounds?
Tracing the history of alloys, we can see that they were used as early as 3000 BCE. So, the first alloy materials used were brass and bronze, which are considered a mixture. The reason is that constituents are physically combined but retain their individual properties.
ProleanTech offers CNC machining services to make the best use of alloy metals. Contact us now to discuss your project and get expert advice.
Characteristics of Alloy Material
Metals are present in various aspects of our daily lives, but pure metals seldom provide the required strength, durability, or resistance for specific applications. This is where the term alloy material comes in. The objective is to improve properties, like strength resistance to corrosion and electricity conduction.
Characteristics of an Alloy
Generally, the factors influencing the key characteristics of an alloy depend on its composition. Yet, one general fact is that alloys are better than pure metals with some major advantages.
Properties of Alloys
Higher Strength – Most alloys are more complex and stronger than pure metals, and thus, they find their applications in converting energy in areas that demand durability. These include construction materials, aircraft, and automotive components.
Corrosion Resistance – Most alloys are far superior to the original pure metals in resisting rust. The stainless steel alloy, with iron, chromium, and nickel, shows no rusting; hence, they are extensively used in kitchen appliances and exposed structures.
Improved Ductility – Many alloys offer good stretching characteristics, enabling their shaping into very fine wires or rolling into sheets without breaking. This characteristic is primarily essential for electrical wiring and metallic foils.
Better Conductivity – Certain alloy types, including brass and copper alloys, make them qualified to be used in electric and electronics applications due to their effective conduction of electricity.
Enhanced Heat Resistance – Many alloys maintain a solid form at a hot temperature. This is very critical in a regime of jet engines or across power plants and heavy industries.
Lighter Weight – Various aluminium alloys provide a good strength-to-weight ratio. These find wide application in air transport and other transport sectors in reducing weight while ensuring strength.
Wear Resistance – Different types of alloys have been specially formulated to resist the wear and tear caused by machines, tools, and heavy-duty equipment.
Hence, all alloy materials are beneficial in almost all industries. They have provided specific properties that make the alloys work much better than pure metals in different applications. The flexibility and these features of alloy materials ensure their place in technology and engineering even in the future.
Read out about CNC machining & its industrial impact to know how it influences the alloys.
Try Prolean Now!
Common Classes of Alloys
Alloy is a miracle when it comes to the effective use of materials. By mixing different metals and sometimes other materials, you may be more comfortable making products of specific strength, durability, and corrosion resistance. Let’s learn about some common alloys to see why they matter.
1. Ferrous Alloys
Assortment of Ferrous Alloys
Those alloys in which at least iron is one and often one of the main alloy components are called ferrous alloys. These have impressive strength and magnetic properties. Some important examples are:
- Steel –This is simply an alloy of iron and carbon, often combined with other metallic elements such as chromium to improve the properties. Steel is, therefore, the most widespread alloy.
- Stainless Steel – Stainless steel is an alloy made with chromium that endows it with a very high degree of rust resistance.
2. Non-Ferrous Alloys
These alloys don’t have iron as a significant constituent, so they are lighter. A few examples are:
Non-Ferrous Alloy Ore Fragments
- Aluminum Alloys – These alloys are light and generally used for aerospace, automotive, and packaging industries.
- Copper Alloys –These include bronze (copper plus tin) and brass (copper plus zinc), which are popular for electrical conduction.
- Titanium Alloys are strong and often used in medicine and aerospace.
3. Superalloys
Superalloy Components: High-Performance Materials
One of the metallic alloy examples is Superalloys. They are used in many applications to respond to extreme environments at extremely high temperatures. The points of superalloy application can be mentioned in jet engines, power plants, and the chemical industry. The base metals in these alloys are nickel, cobalt, or iron. Because of their high resistance to heat and oxidation, they are handy for advanced technology applications.
4. Precious Metal Alloys
These include gold, silver, and platinum because they are precious metals. Their application is seen in jewelry, electrical components, and medical implants. Some prominent metallic alloy examples under this category are:
Gold Ingots – Precious Metal Alloy
- White Gold – Gold mixed with nickel or palladium.
- Sterling Silver – Silver together with copper to make it durable.
- Platinum Alloys – Used for producing high-value jewelry and various industrial applications.
5. Amorphous Alloys (Metallic Glasses)
Amorphous Alloy Spheres With Unique Structure
The atomic arrangement of these alloys is precisely opposite to conventional alloy metal. As a result, they exhibit very high strength and wear resistance. Applications include electronic components, medical devices, and even sporting goods.
6. Shape Memory Alloys
These are the alloys that resume their original shape upon deformation. An example is Nickel-titanium or Nitinol, which has become widely used for medical stents, eyeglass frames, and robotics.
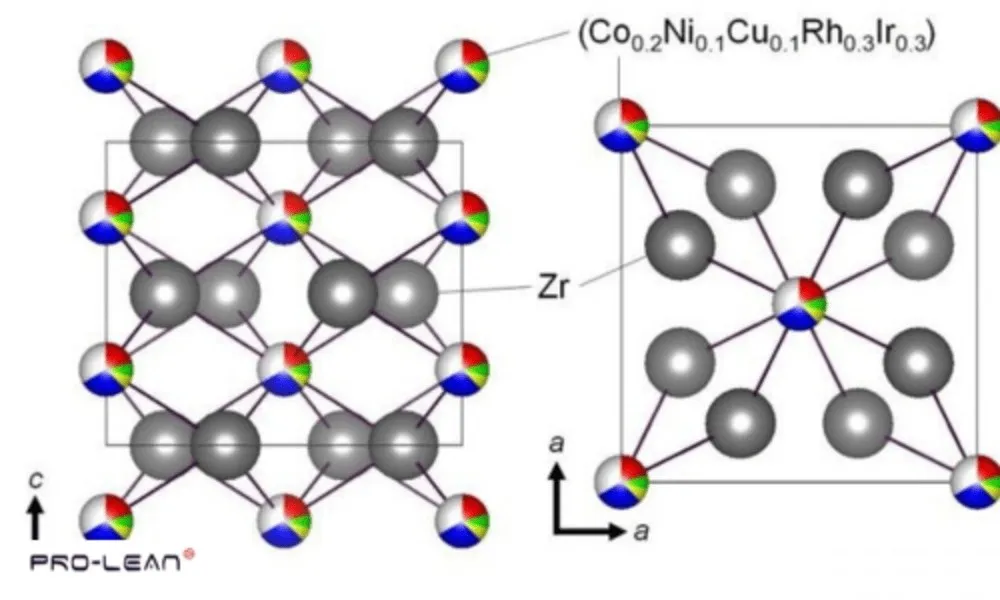
7. High-Entropy Alloys
High Entropy Alloy Atomic Structure
High-entropy alloys are a relatively new class of alloy material that consists of five elements in almost equal amounts. They possess outstanding strength, toughness, and resistance to wear and corrosion, rendering them useful in aerospace and defense applications.
Alloy-making started long ago, and in old age, people used bronze and iron alloys for tools and weapons. Massive changes have occurred in the history of alloys, leading to modern construction, medicine, and technological applications. Now, the composition of an alloy is precisely engineered to meet specific needs, which makes alloys among the best versatile materials in the world.
Mastering Sheet Metal Fabrication is necessary for the proper use of alloys. It ensures precise shaping, cutting, and forming, allowing alloys to reach their full potential in various applications.
Try Prolean Now!
Physical Properties of Alloys
Since alloys are a unique combination of elements, the properties of alloys vary significantly.
Elements of Alloys
Here are the physical properties:
| Alloy | Density (g/cm³) | Melting Point (°C) | Thermal Conductivity (W/m·K) | Electrical Conductivity (% IACS) | Hardness (Brinell) | Tensile Strength (MPa) |
| Steel | 7.85 | 1370–1510 | 16–54 | 10–15 | 120–220 | 400–1200 |
| Aluminum Alloys | 2.7 | 463–671 | 120–235 | 30–60 | 35–160 | 150–550 |
| Brass | 8.4–8.7 | 900–940 | 109–125 | 28–40 | 55–100 | 250–500 |
| Bronze | 8.7–8.9 | 950–1050 | 50–60 | 8–15 | 60–210 | 200–600 |
| Titanium Alloys | 4.43 | 1600–1660 | 6–21 | 0.3–1.2 | 200–400 | 900–1200 |
| Nickel Alloys | 8.4–8.9 | 1200–1450 | 10–30 | 1–7 | 150–400 | 600–1100 |
| Magnesium Alloys | 1.74–1.85 | 430–650 | 76–96 | 30–40 | 30–90 | 180–400 |
| Copper Alloys | 8.7–8.94 | 900–1083 | 200–400 | 50–100 | 60–110 | 200–450 |
| Zinc Alloys | 6.6–7.0 | 380–420 | 90–120 | 27–29 | 70–110 | 150–330 |
| Inconel | 8.5 | 1350–1400 | 11–15 | 1–3 | 200–400 | 600–1200 |
Chemical Properties of Alloy
Likewise, the chemical properties of alloy materials are different. So, here is an overview of the chemical properties of alloy metals:
| Alloy | Corrosion Resistance | Oxidation Resistance | Acid Resistance | Base Resistance | Reactivity | Passivation Behavior |
| Steel | Moderate (depends on alloying elements like chromium) | Good with protective coatings (stainless steel is excellent) | Poor in strong acids (except stainless) | Moderate | High for carbon steel, low for stainless steel | Yes, in stainless steel (chromium forms oxide layer) |
| Aluminum Alloys | Excellent in atmospheric conditions | Good up to ~400°C | Poor in strong acids | Excellent in mild bases | High (reactive with oxygen and acids) | Yes, forms aluminum oxide layer |
| Brass | Moderate (susceptible to dezincification) | Moderate | Poor | Moderate | Moderate (zinc content increases reactivity) | No |
| Bronze | Good (better than brass) | Moderate | Moderate | Good | Low | No |
| Titanium Alloys | Excellent | Excellent | Excellent | Excellent | Low | Yes, forms a protective oxide layer |
| Nickel Alloys | Excellent (used in harsh environments) | Excellent | Excellent | Good | Low | Yes, forms a nickel oxide layer |
| Magnesium Alloys | Poor | Poor (oxidizes easily) | Poor | Poor | Very high (reactive with air and water) | No |
| Copper Alloys | Good (copper naturally resists corrosion) | Moderate | Poor in oxidizing acids | Moderate | Low | No |
| Zinc Alloys | Moderate | Poor | Poor | Poor | High | No |
| Inconel | Excellent (used in extreme environments) | Excellent | Excellent | Excellent | Very low | Yes, forms a chromium oxide layer |
Usage of Alloy Material Based on Industries
Due to their unique properties, the alloy has applications in different industries. Sheet Metal Fabrication Services From China are crucial in utilizing alloys for diverse applications. These services ensure high-quality precision and cost-effective production, making them ideal for the automotive, aerospace, and electronics industries.
Alloy Components in Industrial Applications
Here are the uses of alloys:
- Steel and cast iron are used in the construction industry for structural bases.
- Aluminum and Titanium alloys are used in the aerospace industry to make corrosion-resistant parts.
- Steel and aluminum are used to make the frames of automobiles.
- Titanium and stain steel are used to make surgical instruments.
- Pewter and brass are commonly used in decorative items and jewelry making.
- Copper and aluminum alloys are common for electric wiring. Besides this, they are commonly used in power transmission lines.
Limitations
Although metallic alloys set an example, their usage has certain limitations, too.
- They are often costly due to the scarcity of metals.
- Not all alloys are corrosion-resistant, which limits their usage.
- Producing and shaping alloys requires specialized equipment and techniques, making it time-consuming and resource-intensive.
- Like cast iron, certain alloys can be brittle and prone to cracking under sudden impact or stress.
Wrap Up
That’s all you need to know about alloy material and their properties. At ProleanTech, we offer various alloys tailored to your prototyping and production needs, backed by our top-notch CNC machining services.
Whether you’re looking for aluminum, steel, stainless steel, titanium, or other popular options, we’ve got you covered. Simply upload your CAD files to explore our full range of materials and get an instant quote today!
FAQs
Q1. Why are alloys important?
As compared to pure metal, alloys have high strength and hardness that make them ideal to use for different purposes. Therefore, they are considered important in almost every industry, from aerospace to medical and construction.
Q2. What properties must an alloy used inside the body have?
The human body has a complex environment, so the alloy used inside the body should be biocompatible. It must be non-toxic, corrosion-resistant, and can withstand high stress.
Q3. Does alloy material rust?
Usually, alloy material does not rust easily because of the unique combination of metals. However, certain alloy metals like magnesium alloys can corrode in moist environments.
Q4. Is alloy metal or aluminum? What are the pros and cons of aluminum alloy?
Aluminum is a metal that can be alloyed with other elements to enhance its properties. Aluminum alloy has good corrosion resistance. However, it’s expensive.
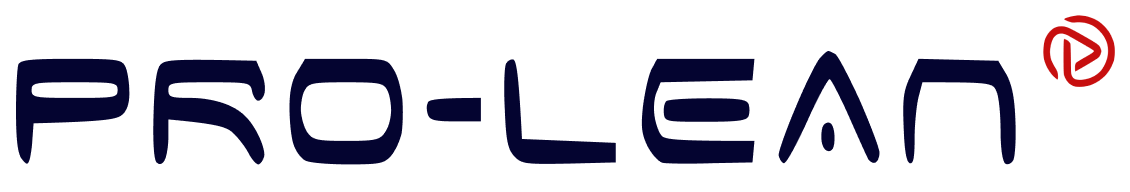









0 Comments