
zinc material feature image
Zinc is a corrosion-resistant metal found in nature in the form of Sphalerite(ZnS), Zinc carbonate(Sphalerite), and calamine. In fact, it is the 24th most abundant and the 4th most widely used material. The use of zinc and its alloys by humans dates back 2,500 years, and yet it remains one of the key metals across modern industries.
You can easily notice the use of zinc in vehicles, electrical systems, architectural elements, galvanized coating, etc. These items are created by CNC machining, casting, or sheet metal fabrication.
Understanding various aspects of zinc material can help you choose the right alloy for your next manufacturing project. Let’s discuss its properties, alloy types, machinability, and many more.
What is Meant by a Zinc Material?

Zinc metal
The term “Zinc material” is not limited to pure metal form, but also includes an extensive range of zinc alloys. It means either pure zinc or any material with a majority percentage of zinc in the composition.
Zinc is symbolized by “Zn” and its position is “30” in the periodic table. Zinc and its alloys provide good electrical, mechanical, and chemical properties. Due to its natural corrosion resistance, zinc is also used to coat different materials through galvanization. Additionally, the appearance is a bluish pale grey, which aids the aesthetic benefits.
Manufacturing techniques such as CNC machining, die casting, and sheet metal work are used to process raw zinc materials into a final part or product.
Brief History of Zinc
Although early traces of use can be seen in ancient Greek during the Bronze Age, some meaningful progress can be seen at 200 BCE by the Romans as they developed an efficient way to extract from oxide ores and blend it with copper to form brass.
In the 4th-3rd century BC, Zinc also started to be extracted in India. Then, a while in history, the start of large-scale extraction and production began in the 15th century in China. After that, it gained popularity for industrial applications, and Zinc Coating for iron & steel during the late 18th century.
Physical, Mechanical, and Chemical Properties of Zinc
Zinc is a pale gray material with a melting point of 419.5 °C, and zinc density is 7.134 g/cm³. It exhibits excellent corrosion resistance, good electrical conductivity, malleability, and ductility. Generally, the zinc remains in the +2 oxidation state and mildly interacts with acids and alkalis.
Now, let’s look at the various properties and facts of zinc.
Zinc Physical Properties
The zinc contains hexagonal crystal structures with packed layers, and the appearance is pale grey, which looks like silver. It has low melting and boiling points, which benefits the casting but limits the thermal applications. The list below outlines other physical characteristics of zinc.
- Metallic Structure: Hexagonal close-packed, space group P6₃/mmc
- Density: 7.134 g/cm³ at room temperature.
- Melting Point: 419.5 °C
- Boiling Point: 907 °C
- Surface Texture: Shiny bluish-white
- Thermal Conductivity: ~120 W·m⁻¹·K⁻¹ at 25 °C
Zinc Mechanical Properties

Galvanization
Pure zinc is a soft metal with comparatively low tensile strength and Young’s modulus of elasticity. However, tensile strength increases to 350MPA in Zamak 2( Zn–Al–Cu) alloy composition. Furthermore, it offers good malleability and ductility.
The list below outlines the key mechanical characteristics of zinc.
| Properties | Typical Range at Room Temp. |
| Tensile strength | 90 – 200 MPa |
| Young’s modulus | ≈ 87 – 110 GPa (commonly cited value ≈108 GPa) |
| Ductility | ≈ 0.3% – 38% (typical commercial ranges: a few % for cast; very high reported for certain rolled forms) |
| Malleability | Ductile / easily worked between ~100–150 °C |
| Compressive strength | 70 – 200 MPa |
| Fatigue strength | lower than steels/Al alloys< 30–50 MPa at 10⁷ cycles (pure Zn) |
Zinc Chemical Properties
Firstly, the zinc material surface itself acts as a natural barrier to corrosion. It reacts with oxygen and forms an oxide layer, and then further reacts with carbon dioxide to form carbonate and oxide film. This protective film prevents rusting and surface degradation.
Zn+O2+2H2O→Zn(OH)
Zn(OH)2→ZnO+H2O
ZnO+2CO2+3H2O→ZnCO3⋅4Zn(OH)2
Next, its reactivity towards acids and alkalis is mild, forming zinc salts with acids and zincate with alkalies.
Try Prolean Now!
Different Types of Zinc Alloys
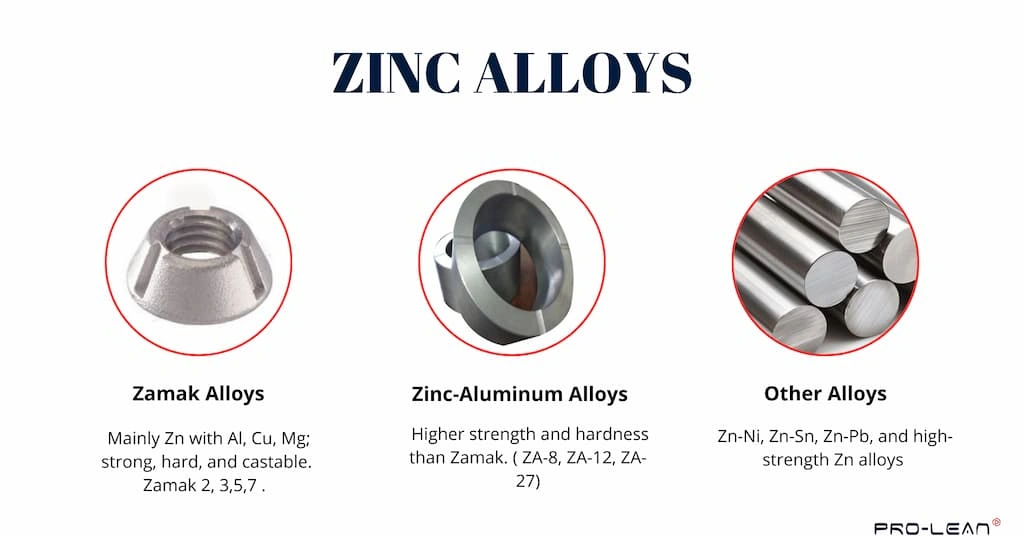
Types of zinc alloys
Besides pure zinc, Zamak 2, Zamak 3, Zamak 5, and Zamak 7 are the common types of zinc alloys. All of these fall under the Zamak series and contain varying percentages of Aluminum, magnesium, and copper. Consequently, Zinc-aluminum alloys are also popular across the machine shops.
1. Zamak Alloys
A typical composition in Zamak alloys involves 94–96% of Zn, 3.5–4.3% of Al, 0.03–1.25% of Cu, and 0.02–0.05%. The aluminum in the Zamak alloys increases the strength, hardness, and strength-to-weight ratio. Copper further enhances the wear and corrosion resistance, whereas magnesium aids in flowability for casting or molding.
Zamak 2 has more hardness and strength than other grades. Zamak 3 provides the highest flowability for casting, whereas Zamak 5 contains extra copper that increases hardness and abrasion resistance.
2. Zinc-Aluminum Alloys
These alloys exhibit even higher tensile strength and hardness than Zamak alloys. The high percentage of aluminum in the composition enhances the physical and mechanical properties of pure zinc metal. The common types of zinc-aluminum alloys are ZA-8, ZA-12, and ZA-27, each indicating the percentage of aluminum in the name.
One main disadvantage of ZA alloys is that added aluminum decreases the flowability, which is a big concern in the die-casting process. Otherwise, it does not affect anything in CNC machining processes. For a comparative view, you can further read ‘Zinc Alloy vs Aluminum’.
3. Other Zinc Alloys

Zinc alloy products
Other than ZA and Zamak, other alloys, such as zinc-nickel, zinc-tin, zinc-lead, and High-strength zinc alloys, are used in manufacturing. Lead-based alloys enhance machinability, Zn-nickel improves mechanical properties, and high-strength alloys provide the desired strength and robustness for structural and automotive items.
Manufacturing Processes for Zinc Parts & Products
Various manufacturing processes are used to produce zinc parts and products, including zinc machining, die casting, sheet metal work, and 3D printing. However, CNC machining and casting are two dominant methods.
Let’s discuss these in more detail.
Zinc Die Casting

Zinc die casting mold
The die casting process involves injecting the molten zinc into a pre-fabricated mold called a “die”. Then, it flows inside the mold cavity and gains the exterior outline on solidification. The casting takes place at high pressure, 1000 to 2000 psi. Meanwhile, the mold is made with tool steel or cast iron by machining it precisely.
This method is suitable for producing zinc items at high volumes with excellent repeatability. The reusable die also makes the process cost-effective. Zinc die casting has numerous applications across various sectors.
Some notable applications include door handles, automotive interior trim parts, underhood components, electronics housings, and decorative hardware. How does it compare with aluminum die casting? You can read Zinc Die Casting vs Aluminum Die Casting here.
Zinc CNC Machining 
Zinc CNC machining
Machining is more suitable for complex and accurate zinc parts. It is also cost-efficient for prototyping. The process involves creating a desired shape from a zinc block, bar, rod, or any other workpiece by removing material. Based on the geometry and complexity, milling, turning, drilling, threading, grinding, and other processes are used.
For CNC machining, you first need to create a CAD model using design software such as SolidWorks and AutoCAD, followed by corresponding CNC programming ( G&M codes). Then, the CNC machine runs the code and controls the movement of the tool/work into a fixed path for material removal. Due to this automation, it enables the manufacturing of accurate custom parts with tolerances as low as ±0.01 mm.
Zamak and ZA alloys are suitable choices for zinc machining projects as they show good machinability. Some application examples of CNC machined zinc parts are mechanical busing, electrical connectors, sensor housings, and decorative trims.
Try Prolean Now!
Applications of Zinc Material

Applications of zinc
Currently, almost half of the zinc consumed worldwide goes for galvanization, a process of creating a protective zinc layer on iron, steel, and other metals. Along with this, different zinc alloys are processed to form desired parts using various manufacturing methods.
The common zinc applications can be seen in medical, electronics, automotive, architecture, construction, and marine industries.
1. Galvanization
Zinc strongly bonds with the substrate material to provide a thin corrosion-resistant layer and extends the product’s life by up to 50%. Methods like hot-dip galvanizing, electrogalvanizing, and thermal coating are used for this. For example, hot-dip is used to coat structural beams, guardrails, mechanical fasteners, and conveyors.
2. Automotive Industry
Generally, casting and CNC machining are used to make zinc auto parts. Zinc alloys are mainly used in either decorative items or structural items for low to medium load conditions.
Alloys like Zamak 3, 5, and 7 are cast or machined to make door lock housing in vehicles, steering components, decorative trim, fasteners, and more.
3. Medical Industry
Zinc applications can be seen in the medical field, from defibrillators to stethoscopes. Another interesting fact is that zinc element uses are critical in our immune system also. Moreover, zinc has favorable mechanical properties for some internal fixation and stent applications.
4. Electronics Industry
Zinc is used in energy storage batteries, sensors, and optoelectronics. Besides this, connectors, enclosures, commercial electrodes, and many other items are made with zinc material.
5. Architecture & Construction
Zinc is used to galvanize structural beams, roofing, and architectural sheets. Additionally, products like trims, facades, bathroom hardware, and cladding systems are produced with zinc.
6. Marine Industry
Another industry that greatly benefits from the high corrosion-resistance of zinc is the marine industry. Different marine hardware is coated with zinc to withstand a harsh environment.
Advantages and Disadvantages of Zinc

Zin pros and cons
Zinc is a versatile metal with several benefits, including corrosion protection, diverse alloying compositions, antibacterial properties, and electrical conductivity. At the same time, there are a few limitations too. It has a low hardness, creep resistance, and melting point, and is quite brittle in pure form.
What Are the Advantages of Zinc Metal?
-
- Corrosion Prevention: Zinc prevents material corrosion by developing a carbonate and oxide film at the surface.
- Alloying Options: Zinc can be mixed with copper, aluminum, magnesium, and other metals to improve properties. It has flexibility in alloying composition.
- Excellent Finishing: As machined zinc parts themselves provide a smooth and good aesthetic. In coating, you get a range of zinc-plating colors to choose from as you need.
- Antibacterial: Zinc maintains hygiene, and itself is an antibacterial metal. This is especially beneficial in food processing and medical applications to maintain cleanliness and safety.
- Electrical Conductivity: Zinc is a good electrical conductor, which makes it usable for electrical connections like connectors and other similar items.
- Recyclability: Zinc can be recycled by melting with a high recovery rate, which makes it an environmentally friendly option.
What Are the Disadvantages of Zinc Metal?
- Low Melting Point: Zinc has a low melting point and can not be used in heat-sensitive applications.
- Brittleness: Pure zinc is brittle in nature and shows a low tensile resistance.
- Chemical Reactivity: Zic reacts with acids & alkalis, causing material degradation. So, it is not fit for acidic and alkaline conditions.
Read more: Difference Between Chrome Plating, Zinc Plating, and Nickel Plating
Custom Zinc Parts at ProleanTech: Die Casting and CNC Machining Services
We have discussed many types and applications of zinc, but you need a manufacturing partner to convert raw zinc material into functional parts and products. You need to look for a few criteria while choosing a reliable partner, including production capabilities, industry experience, availability of materials, and budget.
ProleanTech might fit your criteria in terms of quality and cost among different suppliers and manufacturers. We offer zinc die casting and CNC machining services based on your design.
Our team has a decade of experience handling prototyping and production for multiple industries. You can get support, from material selection to post-processing and final surface treatment. Upload your design and get a quote before starting your project.
Conclusion
This article discusses many aspects of zinc, from its alloys to its applications and manufacturing methods. Understanding the zinc properties and alloy types can help you make better decisions in material selection and which manufacturing method to use. At the end, the final result of the part or product depends on how accurately you choose the right alloy, machining method, and manufacturer.
If you have any further doubts regarding how to make your zinc parts more efficient and cost-effective, contact experts at ProleanTech, no matter which part of the world you are in.
FAQs
What Is Zinc?
Zinc is a brittle corrosion-resistant metal, which is denoted by “Zn” and lies in the 30th position of the periodic table.
What is zinc metal used for?
Zinc is primarily used to galvanize steel and iron to prevent corrosion, and different zinc alloys are machined or cast into industrial components.
What is made out of zinc?
Products made from or containing zinc include galvanized pipes, gutters, roofing, brass fittings, die-cast housings, battery casings, sacrificial anodes, and decorative hardware.
What is the easiest metal to melt?
Among the metals found in solid states(Except mercury), cesium is the easiest metal to melt, with an approximate melting point of 28.5°C. If we look at the everyday metal, it is zinc.


0 Comments