During the finishing of the Metal surfaces electroplating and anodizing enhance their quality. Basically, they are standard finishing procedures that help to make better the resilience of corrosion, endurance, and quality of the surface. Therefore it is important to recognize the differences and parallels between them to make the best use of both of these methods.
In this article, we talk about the difference between “Electroplating vs. Anodizing”. It explains the many types that go into these two methods as well as their advantages, drawbacks, and types.
What are the Differences Between Electroplating vs Anodizing?
The most important difference between these two methods is their various procedures. Although the process of electroplating involves covering a metal surface with another metal complex, on the other hand anodizing involves thickening the natural oxide on a metal surface.
Processing Methods
Let’s grab the processing methods of Electroplating vs Anodizing. In Electroplating the metal is known as the substrate and is linked to the cathode. The solution of electroplating is the electrolyte which has metal ions on it. The direct current flows through the solution and causes metal ions to dissolve from the anode and implant on the substrate. There are two unique materials that need to be involved: the substance that needs to be plated and the coating material. Just like in the plating technique involving the beryllium copper and nickel, the plating layer is known as Nickel and the substrate is simply called the beryllium copper.
While Anodizing is applying the electrochemical layer to the metallic surface to create a thin film coating. When electricity is applied to the electrolyte it causes a thin coating formed on the surface of the treated material which serves as the anode. When the aluminum is oxidized An oxide coating of aluminum will form on the workpiece’s surface.
Alumina is known for its chemical strength protection from oxidation and acid rust and its ability to dye colors.
Coating Objects
A large number of metals are plated items. The most frequently used plating metals are nickel, chromium, tin, copper, silver, and gold. Basically, this method is often used for plating like gold, chrome, nickel, etc. The process of Anodizing is a metal surface process that is used to thicken the metal’s natural oxide coating. Commonly used metals like titanium and aluminum are anodized using a suitable electrolyte.
Working Principles
The substance that is processed in anodizing is called the anode. Although the cathode of the substance is used for coating in electroplating. The charge effect of the electroplating process allows the metal anode ions to deposit their electrons on the cathode. The ions are restored immediately after the anode metal dissolves. The process of anodizing is commonly used for aluminum surfaces because of their high reactivity, This quickly creates a strong oxide layer.
Weight Result
The process of anodizing adds a thin layer of roughly 0.0001-0.001 inches to the aluminum surface which is going to increase the weight by a negligible 1% or less. Although electroplating makes the product heavier by depositing around 0.005 inches of thickness, which helps to take place an additional 10% weight on the metal.
Strength
Electroplating helps to increase the tensile capacity of a metal substance by continuously coating other metals on its surface. Based on the coated substance and thickness, electroplating may increase a metal’s tensile strength by up to 20%. The process of anodizing on the other side may also increase the surface characteristics, Just like hardness, and rust resistance. However, it does not significantly improve the strength of a metal.
Try Prolean Now!
What is Anodizing?
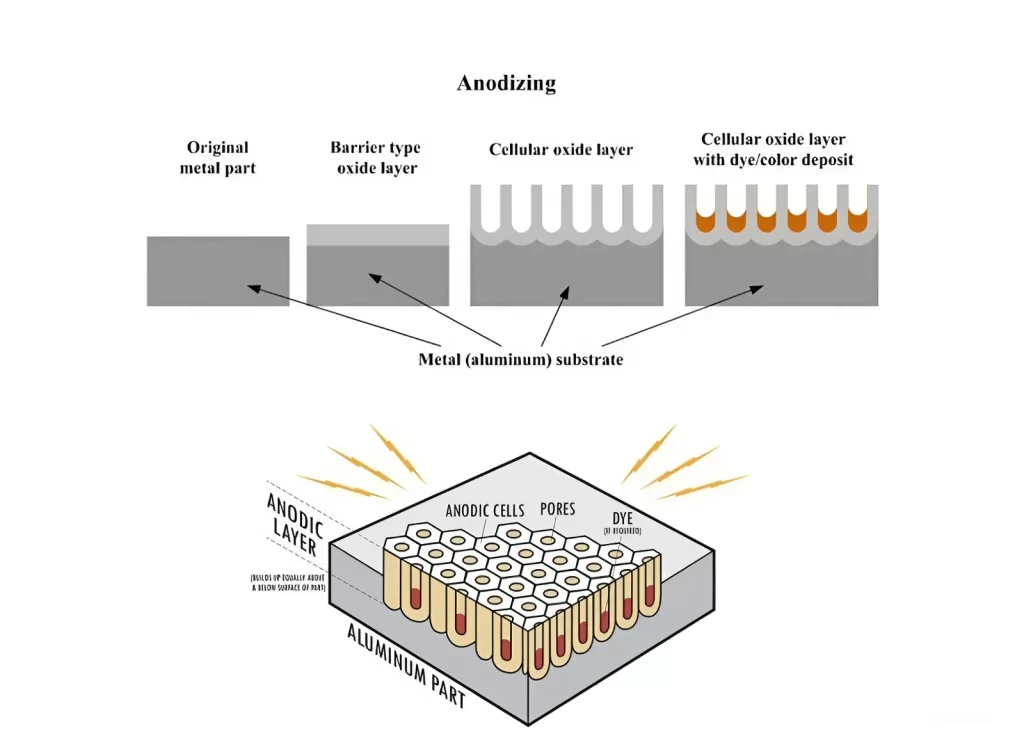
Anodizing is the process of electrochemicals that provide anodic oxide finish to metallic surfaces. Aluminum is the best metal for anodizing, but nonmetallic metals such as titanium anodizing and magnesium can also be treated similarly. The process of aluminum substrate offers the whole aluminum oxide that creates the anodic oxide structure. Instead of being placed topically like paint or plating, this aluminum anodizing is fully merged with the underlying metal substrate, making it resistant to chipping and peeling. Its well-structured permeability structure also facilitates the painting and sealing procedures that are necessary. I hope this concept will help you in learning what is Anodizing.
Anodizing mechanism
Purpose of Anodizing
The process of anodizing services improves the durability, capacity, and structure of metallic material surfaces. The method thickens the layer of natural oxide, preventing wear and corrosion on the metal. Additionally, it helps to increase paint and adhesive adherence, both of which are critical for metal finishing.
Anodizing metals produce colorization and other aesthetic effects, such as decreasing galling in threaded sections. It serves to protect metal surfaces against corrosion by increasing their hardness and thickness. Anodizing is used in industries such as construction, aerospace, and automotive to improve the efficiency, aesthetics, and durability of metal components.
How Does Anodizing Work?
Aluminum alloys are commonly used in anodizing because they oxidize easily, allowing for better control over the growth of oxide layers.
The anodizing process
First, the metal is cleaned and etched to remove imperfections. If required, the metal is electrochemically colored to deposit colored ions in its pores, which is a secondary step in the process. The metallic component (anode) is subsequently exposed to an electric current, which oxidizes its surface. Whenever the electrical charge goes by, the metallic surface interacts with oxygen ions in the solution, forming an oxide layer on the metal (Al2O3 or TiO2). The layers continue to form as more ions are deposited, increasing in thickness. Following this, the porosity increases, allowing dye and other chemicals to be deposited.
The last step of anodizing is to close the gaps with warm water in order to get a long-lasting, non-porous surface. This results in a non-conductive, anodized item that resists wear and corrosion.
Types of Anodizing Process
This is an outline of the many forms of anodizing process:
Chromic Acid
Chromic acid anodizing (Type I) has the lightest surface of the three major anodizing processes, with a layer of oxide density ranging from 0.00002 to 0.0001 inches. Aluminum can survive rusting almost the same when sealed correctly, due to the oxide layer formed during chromic acid anodization.
The first type of oxide coating has a grayish tint and acquires less color when dyed due to the thinner density of the coating layer. The grayish cast limits the application of chromic acid anodizing as a decorative finish.
Some of the primary benefits of chromic acid anodizing are its electronically non-conductive properties as well as powerful adhesion adhesion to other things. Chromic acid anodizing is often used to prepare welded elements and aeronautical materials for subsequent painting.
Anodizing with Sulfuric Acid
The most common anodizing process is Type II, often known as sulphuric acid. The sulphuric acid anodizing technique produces layers approximately 0.0002 and 0.001 inches thin. The oxide deposit alters the part’s surface, making it appropriate for applications requiring resistance to abrasion and durability.
Utilizing the porous nature of sulphuric acid the coatings before closing results in a colorful finished surface on aluminum and similar metals. Aluminum oxide is porous and rapidly absorbs dyes. After the colored substance has been applied, sealing the anodized oxide coating helps to prevent color fade while the component is in use. This anodizing procedure allows you to pick from a number of colors, including red, black, gray, and brown; these are often considered anodize colors.
Sulphuric acid anodizing is less costly and requires a shorter period to reach the thickness you want than other anodizing methods. Type II anodizing is commonly applied to optically and electronic parts, hydraulic valves, body parts, and computer and electronic containers.
Hard Coat Anodizing or Hard Anodize
Hard coat anodizing is often applied using a sulphuric acid-based electrolyte. Compared to sulphuric acid anodizing, it produces a substantially thicker and denser oxide layer.
Hard anodizing normally results in a coating that varies from 0.0005 to 0.002 inch. The severe anodizing method is recommended for situations that require excellent resistance to abrasion in corrosive environments. It may also be useful in circumstances requiring better electrical insulation. Because they may thicken with numerous layers of oxide testimony, Type III anodized coatings aid in the remanufacturing of out-of-spec equipment and the refurbishment of old coatings.
Types of Metals Suitable for Anodizing
Aluminum and aluminum alloys are among the most common anodized materials, but titanium, manganese, magnesium, zinc, and stainless steel are materials that can also be anodized. The surface of anodized aluminum is three times more robust than that of regular aluminum and remains indestructible even after coloring.
Advantages of Anodizing
- Protects the product from corrosion: The additional coating of material generated by anodizing helps to prevent rust and corrosion on metals, extending their lifespan.
- Enhances durability: Anodising increases the wear resistance of the metal’s surface, improving the object’s longevity.
- Enhances the appearance: Anodisation changes the chemical composition of the metal, making its surface more conducive to painting and dyeing.
Limitations of Anodizing
- Visible touch-up: Anodised colored coatings are not resistant to scratches. These scrapes are evident to the touch.
- Limited color selection: The color spectrum of an anodized metal is severely limited by the chemicals employed in the anodizing process. As a result, there are fewer and fewer color possibilities available, giving the metal little alternatives for paint or dying.
Common Anodizing Applications
Anodizing enhances the object’s appearance, and this benefit, together with its endurance, makes the technique applicable to a wide range of businesses.
Construction Industry
Anodized goods such as aluminum railing systems, grilling canopies, and trellises are employed in the building area. Since coated aluminum rusts, the anodized treatment extends the build’s lifespan. Furthermore, anodized items do not deteriorate when exposed to seawater or UV light.
Aerospace Industry
Aluminum finishes are applied on aluminum extrusions, such as trusses, in the aerospace industry to prevent rust and material degradation. Trusses with an anodized finish provide far better refractive and thermal management.
Medical Industry
Anodizing aids in the production of medical equipment that serves a variety of functions in the medical industry. For instance, the majority of anodized items are used in medical devices as replacements for joints and surgical tools.
Automotive Industry
Anodizing is used in the automobile industry to improve engine blocks and wheel corrosion resistance. It also gives external grills, handles, and trimmings a more appealing finish, which improves their appearance.
Try Prolean Now!
What is Electroplating?
Are you trying to understand what is electroplating? Let’s grab the concept hard coat. A plating layer of one metal is placed on top of another metal, known as the substrate, in the electroplating process. The substrate’s conductivity, appearance, corrosion resistance, and aesthetic appeal can all be improved by this technique.
Electroplating process
The following metals can be electroplated: nickel, gold, silver, copper, chromium, and zinc. Furthermore, metals are often electroplated and utilized in the jewelry, automobile, and aerospace sectors to enhance the functioning of the substrate. It can be applied to a metal to increase its acceptance rate for further protective coatings to stop wear and corrosion.
Purpose of Electroplating
A thin layer of metal can be applied by electroplating to a conductive surface to enhance its conductivity, resilience to corrosion, durability, or appearance. This may be necessary to meet specific functional or aesthetic requirements in industries including the jewelry, electronics, and automobile production sectors.
A metal’s physical characteristics can also be enhanced via the electroplating process. It provides corrosion resistance, extending the lifespan of metal components used in several industries. Because electroplating enhances conductivity, it is perfect for electrical and electronic purposes. Additionally, it has a gorgeous finish that enhances the visual appeal of objects like jewelry and automobile trim.
How Does Electroplating Work?
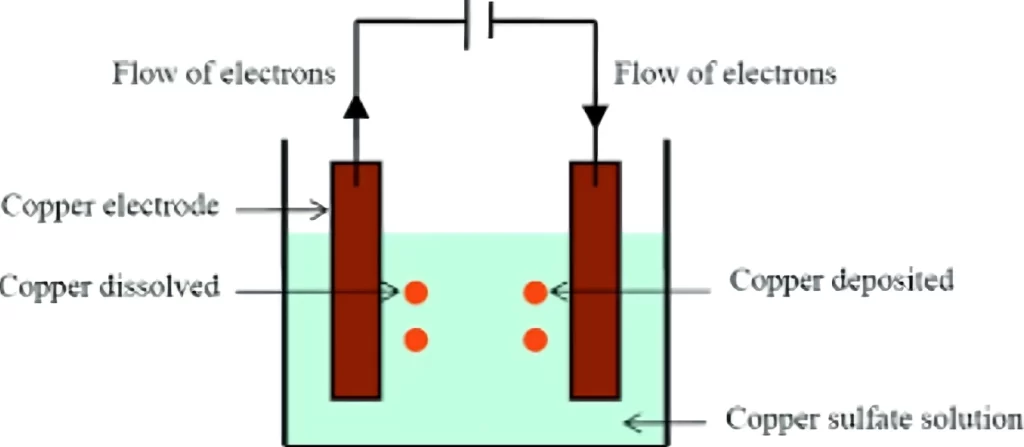
An electrolytic cell applies an ionic metal coating to a conducting surface through the electrochemical process of electroplating. Before being immersed in an electrolytic solution, the substrate—a metal object—is cleaned in preparation for the deposition process.
Subsequently, the solution is mixed with direct current, with the plating material linked to the anode and the substrate being attached to the cathode. As the current runs, metal ions dissolve from the anode into the solution of water and deposit on the metal that is attached to the cathode.
As a result, the plating substance is evenly yet thinly coated on the metal. It is possible to control the electrolyte composition, density, and plating time to affect the layer thickness.
Types of Electroplating Techniques
There are several forms of electroplating, and each one is tailored to a particular material and use. The kinds of electroplating are as follows:
Nickel Plating
Given that it regularly forms connections with copper and aluminum, this kind of plating can act as the underplating for chromium. Utilizing an alloy of nickel and phosphorus during the electroless nickel plating process readily increases hardness and resistance to corrosion. Both functionally and aesthetically, it is used in a variety of sectors, including electronics, automotive, and aerospace.
Chromium Plating
This sort of plating involves applying chromium to the surface of another metal component to enhance its look. Most appealing automobile components in the automotive industry employ chromium plating because of its ability to offer a shining finish and restore tolerances on damaged parts.
Copper Plating
This entails electroplating metal items with a coating made of copper. It is commonly used on metals for electronic parts and is the ideal preparatory coating for other kinds of metal plating. Printed circuit boards, electrical industries, and electronic components are good candidates for copper plating.
Gold Plating
A tiny amount of gold is deposited on the surface of another metal component during the gold-plating process. It is used in the electronics, jewelry, and decorative arts sectors.
Different Metals Suitable for Electroplating
The purpose and necessary material finish determine which metals are utilized in electroplating techniques. Zinc, gold, copper, nickel, chromium, cadmium, silver, zinc, and brass are a few of the common ones. Every metal has distinct qualities and attributes that contribute to the final product.
Zinc, for instance, is a less expensive substitute that aids in halting corrosion in steel and iron components. The use of chromium in plating imparts a glossy appearance to the substrate while preserving its anti-corrosive properties.
Advantages of Electroplating
- By applying a substrate to a metal appearance, electroplating creates a coating that guards against deterioration or corrosion;
- It extends the life of a metal or material and enhances its usefulness;
- A metal material’s ability to conduct electricity can be improved by coating it;
- When metallic components are combined during the electroplating process, metal items’ ornamental finishes are improved, giving them a more appealing look.
Limitations of Electroplating
- Electroplating is a costly procedure since it requires several metal elements, chemicals, and other expensive components;
- The process becomes sluggish and drawn out as additional metal layers and components are applied to the metal surfaces;
- Pollution is caused by the process, which releases dangerous elements into the environment.
Similarities between Electroplating and Anodizing
So we have read about differences in Electroplating vs Anodizing. Perhaps There are several parallels between the two metal coating methods, even if there are also many variances. The fact that they are both electrochemical processes utilizing chemical substrates is one of their apparent commonalities. Furthermore, both anodizing and electroplating involve the deposition of a material on the surface of a metal because they are coating processes.
An anode and a cathode are present in an electrolytic solution in both procedures. These procedures are also used to improve the functioning and look of metal products. The processes of anodizing and electroplating enhance resistance to wear, corrosion, and damage while depositing metals and oxidative layers.
Try Prolean Now!
Prolean Offers One-stop Surface Finishing Services
For your machining tasks, Prolean offers complete electroplating and anodizing services. We also offer creating art, sandblasting coating with powder, polishing, brushing, and other services. Prolean provides superior post-finishing solutions with an emphasis on quality control and customization, enabling your products to meet both practical and aesthetic production standards.
Conclusion
The choice of electroplating vs. anodizing will always be based on the final product’s function and the material in issue. It all depends on your needs and tastes; both metal coating methods have advantages and disadvantages. Furthermore, it is important to give priority to the metal’s nature while comparing the two procedures.
FAQs
Are surfaces that have been electroplated or anodized more durable?
Because anodized surfaces have a protective oxide coating, they are naturally more corrosion-resistant. Surfaces that have been electroplated resist wear better.
How should surfaces that have been electroplated and anodized be maintained?
The easiest way to maintain surfaces that have been anodized or electroplated is to wash them with soap and water to get rid of any debris, and then wipe them down with a gentle microfiber cloth.






0 Comments